Breath Actuated Delivery
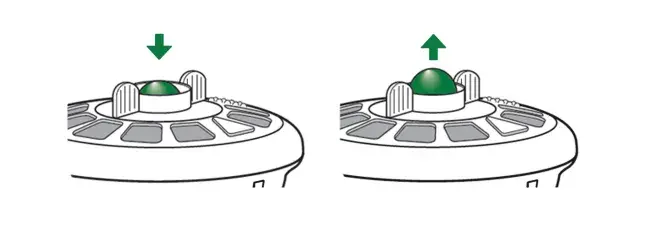
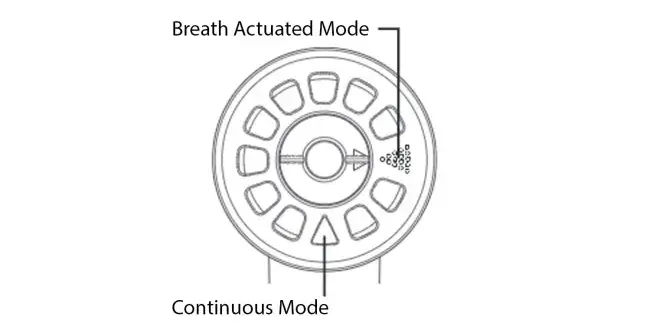
With breath actuated delivery, aerosol is only produced when you breathe in. This means medication is not wasted between breaths or if you need to take a break (for example if you need to cough or have a conversation). This puts you in control of your aerosol treatment. Other nebulizers continuously produce aerosol regardless of whether you are inhaling, exhaling or resting, resulting in medication being lost to the air instead of delivered to your lungs.
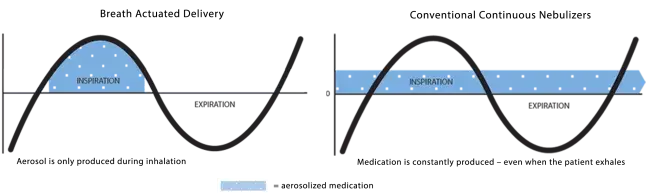
Learn more about the difference breath actuated delivery can make!
FeaturesDesigned with patients in mind
|

|
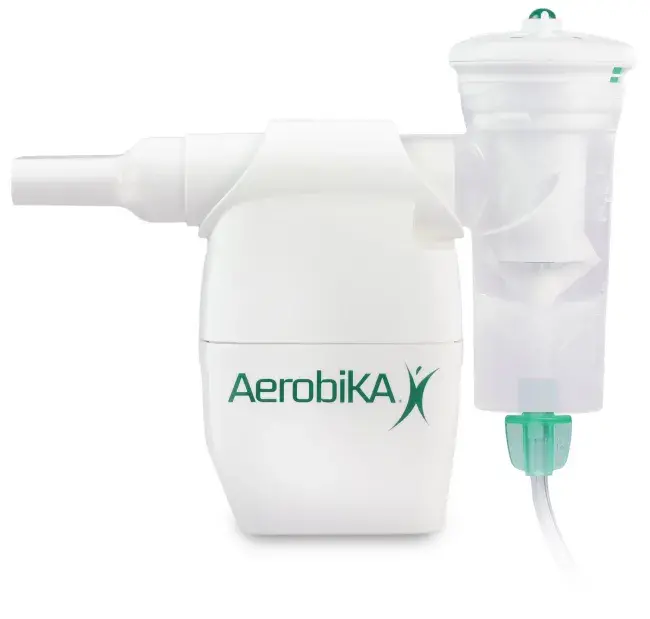
Combined Aerosol and Oscillating Positive Expiratory Pressure (OPEP) Therapy
The AEROECLIPSE* XL BAN* Nebulizer can be connected directly to the AEROBIKA* Oscillating Positive Expiratory Pressure (OPEP) device for nebulizer therapy during inhalation with mucus mobilization during exhalation. Combined treatment could reduce the time needed to take both treatments separately. Speak to your healthcare provider for more information on combination therapy. Download the AEROBIKA* OPEP device instructions for information on how to use the device with the AEROECLIPSE* XL BAN* Nebulizer. |

|