Scientific & Performance Evidence
Trudell Medical International has an enviable history of strong leadership in creating innovative medical devices that enhance the quality of life for people of all ages - all backed by science and clinical evidence.
Breath-Actuated Nebulizers for Asthma and Chronic Obstructive Pulmonary Disease Exacerbation: A Monte Carlo Simulation Demonstrating National Cost Savings and Length of Stay Reduction
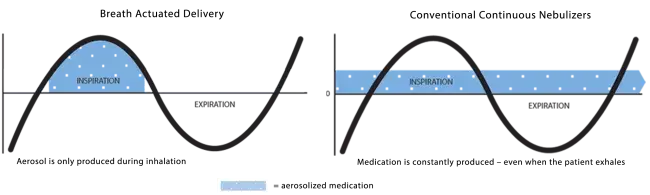
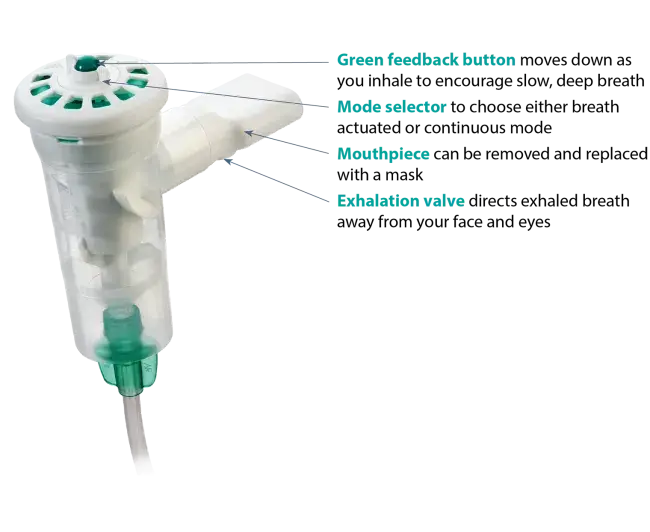
Use of the AEROECLIPSE* II BAN* Nebulizer in the Emergency Department can significantly reduce overall cost of care and improve throughput for asthma and COPD exacerbations.
Download
Efficiency of a Nebulizer Filter Kit to Prevent Environmental Contamination During Nebulizer Therapy
Use the AEROECLIPSE* II BAN* Nebulizer with filter kit to eliminate environment loss of exhaled aerosol.
Download
Drug Delivery Performance and Fugitive Emission Comparison of Two Commercially Available Nebulizer Systems
AEROECLIPSE* II BAN* Nebulizer achieves higher and more consistent delivery than a vibrating mesh nebulizer.
Download
Dose Assurance with Nebulizer Therapy – A Laboratory Investigation into the Medication Delivery Performance of a Range of Different Nebulizers at Different Inspiratory/Expiratory Ratios
AEROECLIPSE* II BAN* Nebulizer achieves high and consistent dose delivery.
Download
A Laboratory-Based Examination of the Potential for Fugitive Emission of Aerosols to the Local Environment from a Range of Commercially Available Nebulizer Systems
AEROECLIPSE* II BAN* Nebulizer substantially reduces environmental losses of aerosolized medication.
Download
Implementation of a BAN* Nebulizer Regimen May Reduce Nosocomial Influenza Acquired by Exposure to Fugitive Droplet Emissions from Continuous Nebulizers Whose Droplets Produced During Exhalation are Vented to the Environment
Implementation of BAN-based therapy has the potential to reduce costs associated with acquisition of nosocomial influenza in the ED.
Download
Performance Evaluation of Four Models of Small Volume Nebulizer
Enhancements to jet nebulizers which allow for increased inspired flow and limiting the cycle for aerosol generation have a positive influence on both delivered medication and particle size fraction delivered to the patient.
Download
AEROECLIPSE* BAN* Nebulizer Clinical Reference Sheet
Performance with commonly prescribed nebulizer formulations.
Download
AEROECLIPSE* BAN* Nebulizer Study Summary
>130 studies with approved nebulizer formulations (including solutions, suspensions and antibiotics) and a variety of novel formulations.
Download