How to Use a Chamber With a Mouthpiece
Follow these simple instructions or download our full instructions ↓.
Based on your language, we suggest to change your settings to: En fonction de votre langue, nous vous suggérons de modifier vos paramètres comme suit :
Use of a spacer is a simple and effective way to help improve the use of your inhaler. Spacers help to ensure that your medicine reaches your lungs where it’s needed and limits the amount that ends up in the back of the throat1.
Follow these simple instructions or download our full instructions ↓.
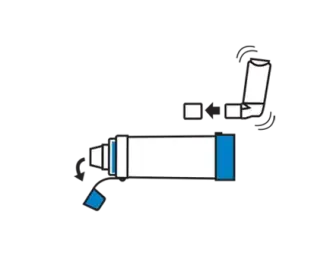
Remove caps from the inhaler and chamber. Shake the inhaler immediately before use as per the instructions supplied with it.

Insert the inhaler into the backpiece of the chamber. Put mouthpiece into mouth and close your lips around it to ensure an effective seal.
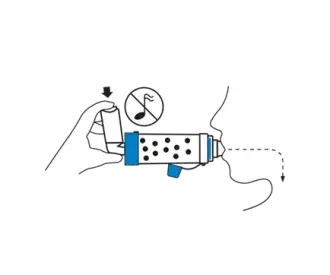
Exhale then press the inhaler once at the beginning of a slow inhalation. Inhale slowly and deeply through the chamber until a full breath has been taken. Hold your breath for 5-10 seconds before exhaling. OR Exhale and press the inhaler once at the beginning of a slow inhalation. Breathe in and out through the chamber for 2-3 breaths keeping lips sealed around chamber mouthpiece.
Follow these simple instructions or download our full instructions.

Remove caps from the inhaler and chamber. Shake the inhaler immediately before use as per the instructions supplied with it.

Insert the inhaler into the backpiece of the chamber. Apply mask to face and ensure an effective seal.
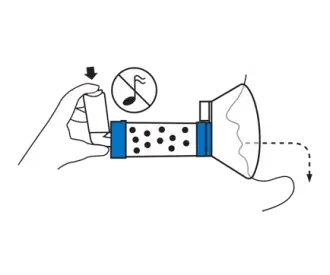
Exhale then press the inhaler once at the beginning of a slow inhalation. Hold mask in place and breathe in and out through the chamber for 5-6 breaths.
Follow these simple instructions or download our full instructions ↓.
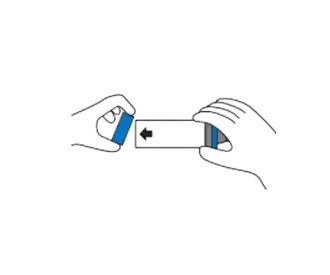
Remove back piece only. (Do not remove mask).
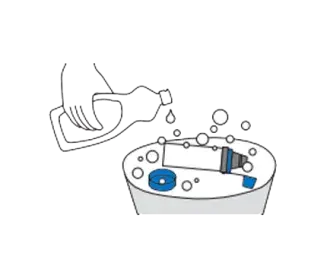
Soak the parts for 15 minutes in a mild solution of liquid dish detergent and lukewarm clean water. Agitate gently. Do not rinse the chamber – leaving a very thin film of detergent enhances the performance of the chamber.
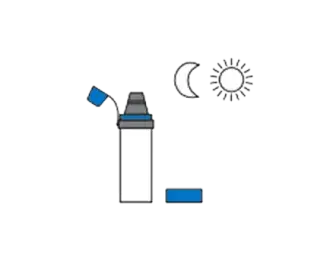
Shake out excess water and allow to air dry in a vertical position. Do not rub dry.
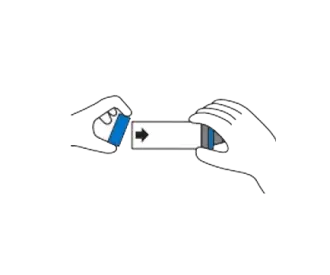
Replace backpiece when unit is completely dry.
The safety and efficacy of AeroChamber Plus® brand of chambers is validated in numerous third-party clinical evaluations with different patient populations with different MDIs.1 It is the most studied brand of spacer; supported by hundreds of in-vitro and in-vivo studies and is listed in the prescribing instructions for most MDIs.
This in vitro study, employing EMA assessment criteria, assessed the equivalence of four anti-static valved holding chambers versus the non-conducting AeroChamber Plus* valved spacer which is most widely referenced in pMDI monographs. Only AeroChamber Plus* Flow-Vu* chamber was equivalent to the reference AeroChamber Plus* device.
The compact Space Chamber†, InspiraChamber† and OptiChamber Diamond† were all inequivalent to the reference chamber. The respirable dose emitted from the chambers was approximately half that of the reference device, confirming that these devices should not be considered interchangeable.
Full Citation: Are valved holding chambers (VHCs) interchangeable? An in vitro evaluation of VHC equivalence. Dissanayake S, Nagel M, Falaschetti E, Suggett J. Pulm Pharmacol Ther. 2018 Feb;48:179-184. doi: 10.1016/j.pupt.2017.10.005. Epub 2017 Oct 10
Examined the effect of AeroChamber Plus* Chamber on lung bioavailability and total systemic exposure of Fostair† in 12 healthy males. Use of AeroChamber Plus* Chamber optimizes the delivery of Fostair† to the lung in subjects with inadequate inhalation technique. The total systemic exposure was not increased, supporting the safety of Fostair† with AeroChamber Plus* Chamber.
Full Citation: Effect of aerochamber plus™ on the lung and systemic bioavailability of beclometasone dipropionate/formoterol pmdi. Singh D, Collarini S, Poli G, Acerbi D, Amadasi A, Rusca A. British Journal of Clinical Pharmacology 2011;72(6):932-9.
44 infants < 2 years were included in this multicenter study evaluating the safety and efficacy of Ventolin† HFA (180 and 360 μg) via AeroChamber Plus* Chamber.
Cumulative dosing with Ventolin† HFA 180 μg or 360 μg via MDI / AeroChamber Plus* Chamber in children younger than 2 years did not result in any significant safety issues and improved asthma symptom score by at least 48%.
Full Citation: Repeat dosing of albuterol via metered-dose inhaler in infants with acute obstructive airway disease – a randomized controlled safety trial. Kaashmiri M, Shepard J, Goodman B, Lincourt WR, Trivedi R, Ellsworth A, Davis AM. Pediatric Emergency Care 2010;26(3):197-202.