A promo code can be entered during the checkout process:
Based on your language, we suggest to change your settings to: En fonction de votre langue, nous vous suggérons de modifier vos paramètres comme suit :
A promo code can be entered during the checkout process:

No, we currently only offer online purchase to addresses within Canada.
Yes, please contact our distribution team for more information.
Many of our products are available for purchase from your neighbourhood pharmacy but we do not have our own store.
London, Ontario, Canada.
In order to cancel your order, please contact customer service.
We use established online-payment providers to facilitate payment (Stripe, PayPal). We do not store or retain payment details.
In order to change your order, please contact customer service.
There are a variety of shipping options and costs that are exposed as you go through the cart. The costs and options depend on where you are shipping to and can be viewed in the checkout process.
There are a variety of shipping options and costs that are exposed as you go through the cart. The costs and options depend on where you are shipping to and can be viewed in the checkout process.
Credit Card, ApplePay, GooglePay, Stripe.
Please contact customer service.
In order to change your order, please contact customer service as soon as possible. We typically ship same day, so we cannot guarantee that we can make the change.
Your payment method will be charged as soon as you complete all steps of the checkout.
The majority of adults do not use their inhalers properly. In fact, a large study showed that 9 out of 10 adults don’t use their inhaler properly1. Chambers help by making it easier to inhale your medication and helping to ensure the puffer medication is delivered to your lungs, where it is needed.
1 Global Initiative for Asthma 2021.
Yes, most adults do not use their inhalers properly. In fact, a large study showed that 9 out of 10 adults don’t use their inhaler properly1.
Chambers (also called spacers) help by making it easier to inhale your medication and helping to ensure the puffer medication is delivered to your lungs, where it is needed.
1 Global Initiative for Asthma 2021.
The majority of adults do not use their inhalers properly. In fact, a large study showed that 9 out of 10 adults don’t use their inhaler properly. 1
Chambers help by making it easier to inhale your medication and helping to ensure the puffer medication is delivered to your lungs, where it is needed.
1 Global Initiative for Asthma 2021.
Your puffer is inserted into your spacer. When the puffer is pressed, the spacer holds the medication spray so that you can breathe the medication in from the spacer, instead of directly from the puffer.
The AEROCHAMBER PLUS* FLOW-VU* Chamber should be cleaned once a week. The Chamber is easy to clean and is dishwasher safe. Wash in lukewarm water and detergent and then rinse and air dry. If cleaning in a dishwasher, ensure the chamber is placed in the top rack of the dishwasher and run on a normal or light cycle, avoiding the heated dry cycle.
No, there is no Latex on the mask or anywhere on the AEROCHAMBER PLUS* FLOW-VU* Chamber.
The average retail price is:
Depending on the province, some chambers are reimbursed. Usually private health plans will cover the cost of the product as part of the extended health benefits. The prescription and paid receipt will need to be sent into the Insurance company following purchase.
Yes. In fact, not only does it work with all MDI’s, the AEROCHAMBER PLUS* brand of chambers is the most recommended by leading metered dose inhaler pharmaceutical companies.
The AEROCHAMBER PLUS* Chamber can also be used with soft mist inhalers.
The AEROCHAMBER PLUS* FLOW-VU* Chamber should be replaced every 12 months. If you live in Canada you are eligible to sign up for our reminder program - for signing up to the program you will receive a free carrying case.
The FLOW-VU* Inhalation Indicator is a valuable feedback tool on the AEROCHAMBER PLUS* FLOW-VU* Chamber that moves as you breathe and helps provide assurance of correct use and medication delivery. The FLOW-VU* Indicator is especially useful for parents who are giving the inhaler medicine to their children. The indicator provides a visual cue of inhalation and correct use for medication delivery.
Yes. The AEROCHAMBER PLUS* FLOW-VU* Chamber can be used with all commonly prescribed metered dose inhalers. The AEROCHAMBER PLUS* brand of chambers are the most recommended by leading metered dose inhaler pharmaceutical companies.
AEROCHAMBER PLUS* FLOW-VU* Chamber is available at the pharmacy counter in pharmacies across Canada.
No, spacers cannot be used with dry powder inhalers.
Yes, you can use an AEROCHAMBER PLUS* FLOW-VU* Chamber to take your Respimat inhaler.
The MyAEROCHAMBER* loyalty program provides a free carrying case for registering – sign up today!
We also have a new product, AEROCHAMBER PLUS* FLOW-VU* Chamber which acts as both a chamber and a carrying case for your inhaler. See if the AEROCHAMBER2GO* device is right for you.
No, infants and young children must use a spacer with a facemask to take their inhaled medication.
Spacers with facemasks are essential when giving inhaled medication to infants and younger children.
The child can breathe normally through the facemask and chamber to inhale the medication.
The FLOW-VU* indicator moves back and forth as the child breathes, helping to provide assurance of correct use and medication delivery.
Yes, but a spacer with a facemask is essential to give an inhaler to infants and younger children. The child can breathe normally through the facemask and chamber to inhale the medication.
The FLOW-VU* indicator moves back and forth as the child breathes, helping to provide assurance of correct use and medication delivery.
Children can typically transition to a mouthpiece around the age of 5 and when they can make a tight lip seal around the mouthpiece.
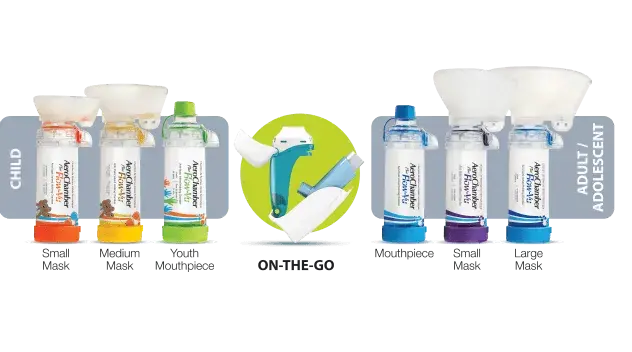
Facemasks are essential for infants and younger children. Facemasks are also ideal for increased ease of use. You simply need to breathe normally through the chamber to inhale the medication.
We have recently introduced a compact spacer called AEROCHAMBER2GO* chamber, which acts as a pocket-sized carrying case for your inhaler that is also an inhaler spacer.
We have recently introduced a compact spacer called AEROCHAMBER2GO* chamber, which acts as a pocket-sized carrying case for your inhaler that is also an inhaler spacer. See if the AEROCHAMBER2GO* chamber is right for you.
No, you can purchase a spacer without a prescription from a pharmacy or online store however reimbursement through private or public insurance often requires a prescription and prescription receipt.
The AEROBIKA* Oscillating PEP device is an airway clearance therapy device used to mobilize excess lung secretions and improve breathing1. It can be used to manage respiratory conditions such as COPD, bronchiectasis and Cystic Fibrosis. You should consult your healthcare professional to see if it is a good fit for you.
1. Jean Bourbeau, R. Andrew McIvor, Hollie M. Devlin & Alan Kaplan (2019): Oscillating positive expiratory pressure (OPEP) device therapy in Canadian respiratory disease management: Review, care gaps and suggestion for use, Canadian Journal of Respiratory, Critical Care, and Sleep Medicine, DOI: 10.1080/24745332.2018.1558426
The AEROBIKA* Oscillating PEP device is not recommended for the following conditions:
You should consult your healthcare professional to see if it is a good fit for you.
The AEROBIKA* OPEP device is drug-free and can be an add-on therapy to your existing medications. You should continue to take your prescribed medications as directed by your healthcare professional and consult your healthcare professional to see if it is a good fit for you.
It is recommended to use the device at least twice daily, once in the morning and once at night or as directed by your healthcare professional. Start with a few minutes, slowly moving up to 10 minutes at a time, as you are comfortable. The AEROBIKA* OPEP device is compact and portable and can easily go with you on your travels.
The device should be replaced after 12 months of use, or immediately if damaged.
The AEROBIKA* OPEP device may be used with a small volume nebulizer with a 22 mm adapter. The AEROECLIPSE* XL BAN is the most efficient option due to the breath actuated function. Combined therapy should be used only if advised by a healthcare provider.
If you need to rest, that is not a problem. The resistance setting can be reduced, or you can reduce the time of the treatment if you cannot complete 10 minutes of therapy. Take a break until you are ready to continue. If you have additional questions, please consult a healthcare professional.
Most prefer to complete their treatment at a specific time in their morning and evening routine. For example, while watching a favourite TV show, or listening to the radio.
Yes, you can use the AEROBIKA* OPEP device.
It is recommended to use AEROBIKA* OPEP device before taking medication but after the completion of other airway clearance therapy, as this may result in clearer airways and better drug deposition. You should consult your healthcare professional regarding the use of the device if you are taking medication.
The AEROBIKA* OPEP device may also be used with a small volume nebulizer with a 22 mm fitting. Your healthcare professional will advise which medication to use for combined treatments. Medications which open the airways (eg. albuterol) or help to thin mucus (eg. hypertonic saline) would be good choices to use with the AEROBIKA* OPEP device because they help to remove or thin the mucus in your lungs.
Caution: Medications to stay in the lungs, like antibiotics, should be taken with the nebulizer alone after completing a treatment with the AEROBIKA* device.
The AEROBIKA* OPEP device resistance setting should be set up by a healthcare professional. It is recommended to start at the middle of the resistance indicator. The goal of using the device is to do so continuously for up to 10 minutes. If this is comfortable and easy to do, then you have the correct resistance. If the exhalation is too easy or doesn’t seem to be loosening up any mucus, then adjust to the higher resistance.
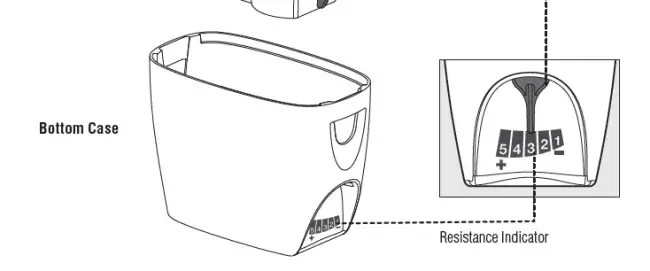
The resistance indicator slides to point to point to the numeric symbol on the device. The numeric symbol “1” is the lowest setting, the numeric symbol “5” is the highest resistance setting.
The Aerobika* OPEP device has 5 resistance settings that adjusts to each user’s capacity. Sliding to the left, towards 5, you experience greater resistances. Moving right, towards 1, lessens than resistance. It is recommended to set the Aerobika* device to resistance level 3, the middle resistance.
An important note with the resistance is that higher does not mean better. Treatment is maximized when the device is used with the resistance set to an appropriate level for the respective user.

When you have excess mucus that builds-up in the lungs, it can result in symptoms. As you exhale through the device, the airways are opened. As the airways open, vibrations help thin and loosen mucus which can then be expelled.
When you have COPD or Chronic Bronchitis, Cystic Fibrosis or Bronchiectasis excess mucus can build-up in the lungs resulting in symptoms. The Aerobika* Oscillating PEP device is an airway clearance therapy device used to mobilize excess lung secretions and improve breathing.
The AEROBIKA* OPEP device is DRUG-FREE and can be an add-on therapy to your existing medications. You should continue to take your prescribed medications as directed by your healthcare professional. You should consult your healthcare professional to see if it is a good fit for you.
We recommend using the device twice daily, once in the morning and once at night. Start a session lasting a couple of minutes. As your comfort level with the device improves, slowly increase the treatment duration to 10-20 minutes at a time.
The AEROBIKA* OPEP device is easy to clean and maintain.
The AEROBIKA* OPEP device can also be cleaned with a dishwasher. Place the device in a basket on the top rack of the dishwasher after disassembly. Full instructions can be found with the included instructions package.
Healthcare professionals should always use clinical judgment to select the appropriate cleaning and disinfecting method and frequency, based on patient needs. If necessary, clean the AEROBIKA* device once daily. Note: Proper and regular cleaning of the device is important for it to work correctly.
A Peak Flow Meter (PFM) is a tool you can use to check how your lungs are doing – to help determine whether you should take your medication or perhaps call your doctor. Just as a thermometer can measure body temperature and give you an indication of a fever, a TRUZONE* peak flow meter measures Peak Expiratory Flow – the fastest speed you can force air from your lungs after taking a deep breath which is a measurement of your lung health. Peak Expiratory Flow readings are higher when you are well and lower when airways are constricted.
Normal Peak Flow Rates vary based on sex, age and height.
These numbers are for reference only since individuals could have a personal best peak flow rate number that may be higher or lower than the average.
Many people underestimate the severity of their asthma. Some may even accept daily symptoms as a ‘normal’ part of asthma. The fact is that daily symptoms are not normal and can be potentially life threatening. Variations in peak flow trends can signal the patient to implement his or her asthma management plan and can alert physicians to adjust treatments to prevent emergency room visits or hospitalizations. Conversely, use of a peak flow meter is also helpful when trying to reduce medications for patients whose asthma is under control.
Anyone 5 and older with asthma could benefit from using a TRUZONE* peak flow meter, as the daily readings can help you and your healthcare professional understand how well your asthma program is working. Your doctor may even make changes to your medications and therapy program based on your peak flow readings, as it may identify changes, trends or patterns in your respiratory symptoms.
The TRUZONE* Peak Flow Meter differs from other peak flow meters in several significant ways: the TRUZONE* Peak Flow Meter ColorZone™ zone management system does not require the patient to make mathematical calculations to establish patient zones, and its logarithmic rather than linear scale simplifies the reading and recording of peak expiratory flow rates at critical low flow areas. In addition, the TRUZONE* Peak Flow Meter features a protected internal flow indicator to prevent tampering and any inadvertent changes to peak flow readings.
TRUZONE* Peak Flow Meter is available at many pharmacy counter in pharmacies across Canada or can be purchased on this website. See "add to cart" above.
You can print another daily record at any time.
The TRUZONE* Peak Flow Meter can be cleaned using the normal cycle of a dishwasher or by hand. To wash by hand, soak the device in warm soapy water, agitating gently. Do not use a brush or other object to clean the inside. Rinse with clean water. Gently shake out excess water and allow to air dry thoroughly in a vertical position.
The device should be cleaned weekly.
Yes, the device should be replaced every 12 months.
No, but we recommend the device be replaced every 12 months.
Yes, the device can be returned to the pharmacy where you purchased the device so that the pharmacist can examine the device. If the device is not working properly, the pharmacist should give you a replacement device and return it to the respective distribution centre.
The chamber can be used with many of the commonly prescribed metered dose inhalers. It does not fit the round style of inhalers such as Airomir, Alvesco or Qvar.
The chamber can be used with many of the commonly prescribed metered dose inhalers. It does not fit the round style of inhalers such as Airomir, Alvesco or Qvar.
We recommend cleaning on a weekly basis.
The chamber can be used directly out of the package and should be cleaned weekly.
OR
The AEROCHAMBER2GO* chamber should be replaced after 12 months of use.
The majority of adults do not use their inhalers properly. In fact, a large study showed that 9 out of 10 adults don’t use their inhaler properly.
Chambers help by making it easier to inhale your medication and helping to ensure the puffer medication is delivered to your lungs, where it is needed.
The chamber was designed for use by adolescents and adults. It is important that you are comfortable with opening and closing the chamber before storing your inhaler inside of it.
We have received confirmation of coverage in Quebec via both provincial and private insurance plans.
Anticipate private payer coverage will be available in August for plans outside Quebec (which already has coverage). Medical plan coverage is dependent on what level of plan the employer has in place for the employee. Devices are often covered on extended health benefits via a manual submissions process. This means that you have to pay up front at the pharmacy and submit the prescription receipt to your insurance carrier.
We have received confirmation of coverage in Quebec via both provincial and private insurance plans.
Anticipate private payer coverage will be available in August for plans outside Quebec (which already has coverage). Medical plan coverage is dependent on what level of plan the employer has in place for the employee. Devices are often covered on extended health benefits via a manual submissions process. This means that you have to pay up front at the pharmacy and submit the prescription receipt to your insurance carrier.
No. It is an integrated dose counter that is supplied as part of the MDI drug product development by the pharmaceutical company and approved with the drug.
Working with pharma partners, Trudell Medical International customizes the AEROCOUNT* Dose Indicator, including the starting count and forces, to suit the inhaler. Trudell can also customize the display printing on the indicator and exterior colours.
Yes, the AEROCOUNT Smart Dose Counter has bluetooth connectivity. Contact us for further information on our connected solutions for extending patient care.
Due to the operational design, no additional force is required to use the MDI when integrated with the AeroCount* Dose Indicator.
The small size mask is recommended for up to 18 months of age. The medium size mask is recommended for 1 to 5 years of age. The large size mask is recommended for over 5 years of age.
Masks are essential for infants and younger children. Masks are also ideal for increased ease of use.
The mask is silicone. It’s not made or manufactured with bisphenol A (BPA), phthalates, latex or lead.
The COMFORTSEAL* Mask should be replaced after 6 months of use.
The mask can be cleaned after each use.
The mask can be disinfected each day.
† trade-marks of the respective companies
The tubing should be replaced with the nebulizer. The AEROECLIPSE* XL BAN* Nebulizer should be replaced after 6 months of use. The AEROECLIPSE* II BAN* Nebulizer should be replaced after 7 days of use.
You may notice condensation on the inside of the EZ TWIST Tubing. This is normal. To remove the condensation, simply connect one end of the tubing to the compressed air supply, and allow the air to run without the nebulizer attached. This will force air through the tubing, drying the inside. To clean the exterior, wipe with a damp cloth. Allow to air dry thoroughly.
EZ TWIST Tubing cannot be disinfected or sterilized.
With breath actuated delivery, aerosol is only produced during the inspiratory cycle. This means medication is not wasted between breaths or during breaks in treatment (for example to cough or have a conversation). This puts the patient in control of their aerosol treatment. Other nebulizers continuously produce aerosol regardless of whether the patient is inhaling, exhaling or resting, resulting in medication being lost to the air instead of delivered to the lungs.
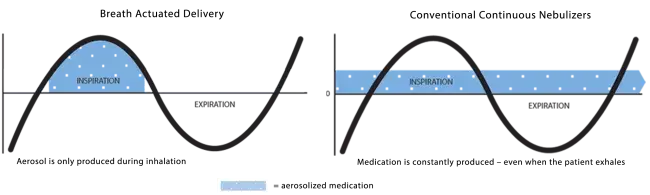
The AEROECLIPSE* II BAN* Nebulizer only produces aerosol during inhalation, so treatment time is dependent on breathing pattern and also breaks taken during the treatment. The dose (or amount of medication) and the gas source used (for example, hospital wall air) are other factors that affect treatment time.
Nebulizer design and the dose or amount of medication it’s delivering affect treatment time. To keep treatment time consistent, it’s important to properly care for the nebulizer according to the device’s instructions for use.
The frequency of nebulizer treatments depends on the type and severity of the patient’s condition. A health care provider can determine what’s right for the patient.
Nebulizers are often selected for patients such as infants and small children. A health care provider can determine what’s right for the patient.
The number of nebulizer treatments depends on the type and severity of the patient’s condition. Treatments may be recommended once or twice to several times daily. A health care provider can determine what’s right for the patient.
The AEROECLIPSE* II BAN* Nebulizer is an innovative device that produces aerosol only during inhalation. Most other nebulizers continuously produce aerosol regardless of whether the patient is inhaling, exhaling or resting. As a result, a large portion of the prescribed medication in a continuous nebulizer is released to the environment and is not inhaled. Care providers and others in the immediate surroundings have the risk of inhaling potentially harmful aerosolized emissions.

The AEROECLIPSE* II BAN* Nebulizer allows the healthcare provider many treatment options that may include fewer or shorter treatment times, less medication put into the nebulizer, or running the AEROECLIPSE* II BAN* Nebulizer continuously.
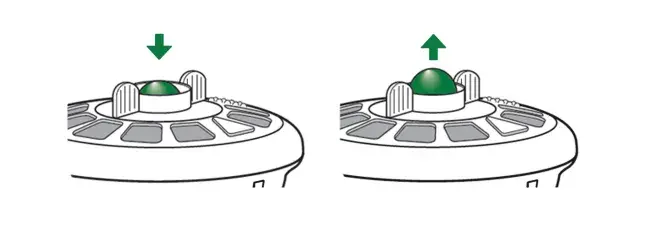
The green feedback button on the top of the AEROECLIPSE* II BAN* Nebulizer lowers during inhalation indicating that aerosol is being produced. When the patient stops inhaling, the button returns back to its original position indicating aerosol production has stopped. This movement can help encourage the patient to take long, slow breaths.
Down Position: Lowers upon inhalation.
Up Position: Rises back to the original ‘up’ position at the end of inhalation or between breaths.
Yes, the mouthpiece on the AEROECLIPSE* II BAN* Nebulizer can be removed and replaced with a COMFORTSEAL* Mask. The silicone mask contours gently to the face, providing a secure and comfortable fit. COMFORTSEAL* Masks are available in small, medium and large sizes.

Refer to the instructions for use for additional information.
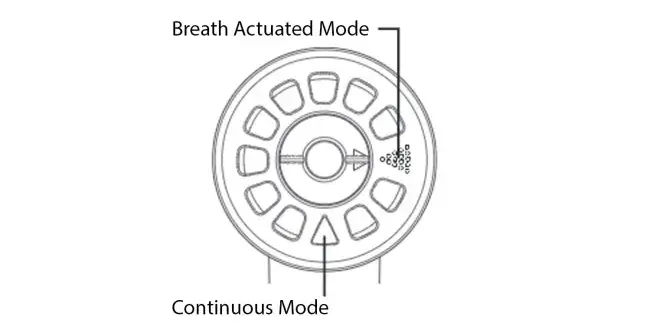
To use the AEROECLIPSE* II BAN* Nebulizer in breath actuated mode, move the mode selector on the top of the nebulizer to the breath actuated position (*). If there is a time when you would rather have a continuous mist, simply move the mode selector clockwise to the continuous mode position (**).
* Breath actuated position:
** Continuous mode position:
Yes, simply use the device in the continuous mode position. Simply move the mode selector clockwise to the continuous mode position (**).
** Continuous mode position:
The AEROECLIPSE* II BAN* Nebulizer should be replaced after 7 days of use.
We recommend cleaning the nebulizer (excluding tubing) after each use.
The nebulizer (excluding tubing) can be cleaned after each use.
Refer to the instructions for use for additional information.
* Breath actuated position:
The nebulizer (excluding tubing) can be disinfected each day. First, follow the cleaning instructions. Do not reassemble the nebulizer. Soak the mouthpiece, nebulizer top and nebulizer cup in 70 % isopropyl alcohol for 5 minutes. Rinse the parts thoroughly with sterile water. Allow the parts to air dry thoroughly before reassembling. After the nebulizer is fully dry and assembled, store in a clean plastic bag or container.
Refer to the instructions for use for additional information.
No, the AEROECLIPSE* II BAN* Nebulizer cannot be sterilized. Do not autoclave.
You may notice condensation on the inside of the EZ TWIST Tubing. This is normal. To remove the condensation, simply connect one end of the tubing to the compressed air supply, and allow the air to run without the nebulizer attached. This will force air through the tubing, drying the inside. To clean the exterior, wipe with a damp cloth. Allow to air dry thoroughly.
Refer to the instructions for use for additional information.
Yes, a filter kit is available for use with AEROECLIPSE* BAN* Nebulizers. The filter kit filters fugitive emissions (aerosolized medication)1, viruses2 and bacteria2 from the patient's exhaled breath. It reduces exposure of healthcare providers to potentially harmful emissions. The filter kit is a single patient, single use accessory (use only once).
| Aerosol Performance: | |
| Aerosol Output1 of AEROECLIPSE* BAN* Nebulizer with Filter Kit compared to the BAN* Nebulizer alone | ≥ 90 % |
| Aerosolized Medication Emissions1 when assembled with AEROECLIPSE* BAN* Nebulizer | ≤ 1 % |
| Filtration Performance: | |
| Viral Filtration Efficiency2 | ≥ 99.99 % |
| Bacterial Filtration Efficiency2 | ≥ 99.999 % |
1. TMI, Data on file 30 August 2020.
2. Breathing Filter Product Report, 24 October 2020, on file TMI.
The AEROECLIPSE* II BAN* Nebulizer can be connected directly to the AEROBIKA* OPEP device for nebulizer therapy during inhalation with secretion mobilization during exhalation. To use, first remove the mouthpiece from the AEROECLIPSE* II BAN* Nebulizer. Then, attach the device to the nebulizer port of the AEROBIKA* OPEP device. No adaptor is required. A healthcare provider can advise which medication to use for combined treatments.
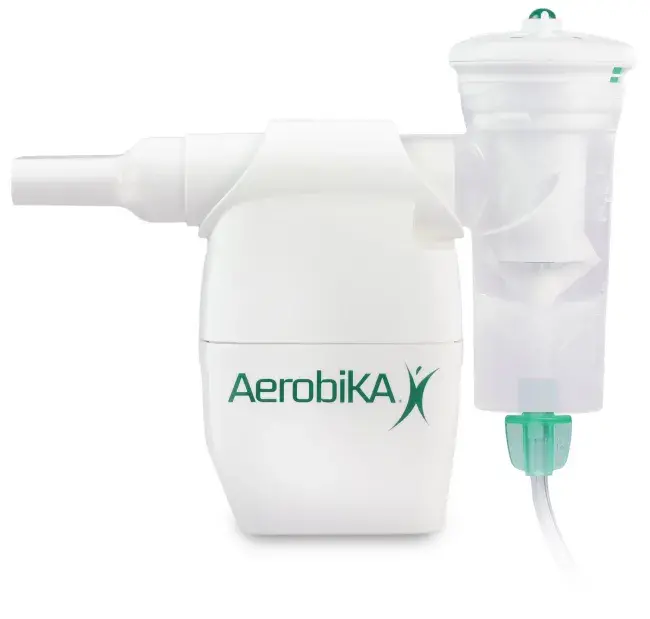
A nebulizer is a medical device designed to work with a compressed air source to convert liquid medication into a fine mist so it can be inhaled directly into the lungs. You may need nebulizer treatments if you have lung disease such as cystic fibrosis, bronchiectasis or COPD (chronic obstructive pulmonary disease). If you have questions about the prescribed medication inside the nebulizer, please talk to your healthcare provider.
A nebulizer compressor (also called a nebulizer machine) is a medical device intended to deliver compressed air to a jet or pneumatic nebulizer. Compressors can be electrically, or battery powered.
Your physician may order your inhaled medication to be given with a nebulizer instead of by an inhaler (puffer). Elderly or those unable to operate or coordinate the use of an inhaler may find a nebulizer easier to use. Please talk to your healthcare provider about which drug delivery type is right for you.
With breath actuated delivery, aerosol is only produced when you breathe in. This means medication is not wasted between breaths or if you need to take a break (for example if you need to cough or have a conversation). This puts you in control of your aerosol treatment. Other nebulizers continuously produce aerosol regardless of whether you are inhaling, exhaling or resting, resulting in medication being lost to the air instead of delivered to your lungs.
The AEROECLIPSE* XL BAN* Nebulizer only produces aerosol when you inhale, so treatment time is dependent on your breathing pattern and breaks you might take during your treatment. The dose (or amount of medication) and the gas source you use (for example, compressor) are other factors that affect treatment time.
Nebulizer design and the dose or amount of medication it’s delivering affect treatment time. To keep treatment time consistent, it’s important to properly care for your nebulizer according to the device’s instructions for use.
The frequency of your nebulizer treatments depends on the type and severity of your condition. Please speak with your health care provider to understand what’s right for you.
Nebulizers are often selected for patients such as infants and small children. Please speak with your health care provider to understand what’s right for your child.
Nebulizers convert medication into an aerosol or mist that can be inhaled into your lungs. Nebulizers are used in the treatment of cystic fibrosis (CF), bronchiectasis, chronic obstructive pulmonary disease (COPD), asthma, nontuberculous mycobacterial (NTM) lung disease and other respiratory conditions.
The number of nebulizer treatments depends on the type and severity of your condition. Treatments may be recommended once or twice to several times daily. Please speak with your health care provider to understand what’s right for you.
The AEROECLIPSE* XL BAN* Nebulizer is an innovative device that produces aerosol only when you breathe in. Most other nebulizers continuously produce aerosol regardless of whether you are inhaling, exhaling or resting. As a result, a large portion of the prescribed medication in a continuous nebulizer is released to the environment and is not inhaled. Care providers and others in your immediate surroundings have the risk of inhaling potentially harmful aerosolized emissions.
The green feedback button on the top of the AEROECLIPSE* XL BAN* Nebulizer lowers when you inhale indicating that aerosol is being produced. When you stop inhaling, the button returns back to its original position indicating aerosol production has stopped. This movement can help encourage you to take long, slow breaths.
Down Position: Lowers upon inhalation.
Up Position: Rises back to the original ‘up’ position at the end of inhalation or between breaths.

Yes, the mouthpiece on the AEROECLIPSE* XL BAN* Nebulizer can be removed and replaced with a COMFORTSEAL* Mask. The silicon mask contours gently to your face, providing a secure and comfortable fit. The COMFORTSEAL* Mask is available in small, medium and large sizes.

Refer to the instructions for use for additional information.
To use the AEROECLIPSE* XL BAN* Nebulizer in breath actuated mode, move the mode selector on the top of the nebulizer to the breath actuated position (*). If there is a time when you would rather have a continuous mist, simply move the mode selector clockwise to the continuous mode position (**).
* Breath actuated position:
** Continuous mode position:

Yes, simply use the device in the continuous mode position. Simply move the mode selector clockwise to the continuous mode position (**).
** Continuous mode position:
The AEROECLIPSE* XL BAN* Nebulizer should be replaced after 6 months of use.
We recommend cleaning the nebulizer (excluding tubing) after each treatment.
The nebulizer (excluding tubing) can be cleaned after each use.
Refer to the instructions for use for additional information.
* Breath actuated position:
The nebulizer (excluding tubing) can be disinfected each day. First, follow the cleaning instructions. Do not dry or reassemble the nebulizer. Use any of the following methods to disinfect the nebulizer.
Refer to the instructions for use for additional information.
† trade-marks of the respective companies
You may notice condensation on the inside of the EZ TWIST Tubing. This is normal. To remove the condensation, simply connect one end of the tubing to the compressed air supply, and allow the air to run without the nebulizer attached. This will force air through the tubing, drying the inside. To clean the exterior, wipe with a damp cloth. Allow to air dry thoroughly.
Refer to the instructions for use for additional information.
The AEROECLIPSE* XL BAN* Nebulizer can be used in the hospital with wall air or with a compressor.
Clean, disinfect and sterilize the nebulizer before using it with a different patient. This will prevent cross infection.
The nebulizer (excluding the tubing) can be cleaned, disinfected and sterilized up to 150 times. The tubing must be replaced between patients.
Cleaning and Disinfection
Sterilization
* Breath actuated position:
Figure 1:
† trade-marks of the respective companies
The AEROECLIPSE* XL BAN* Nebulizer can be connected directly to the AEROBIKA* OPEP device for nebulizer therapy during inhalation with secretion mobilization during exhalation. To use, first remove the mouthpiece from your AEROECLIPSE* XL BAN Nebulizer. Then, attach the device to the nebulizer port of the AEROBIKA* OPEP device. No adaptor is required. Your healthcare provider will advise you which medication to use for your combined treatments.
Download the AEROBIKA* OPEP device instructions for information on how to use the device with the AEROECLIPSE* XL BAN* Nebulizer.

Yes, a filter kit is available for use with AEROECLIPSE* BAN* Nebulizers. The filter kit filters fugitive emissions (aerosolized medication)1, viruses2 and bacteria2 from the patient's exhaled breath. It reduces exposure of healthcare providers to potentially harmful emissions. The filter kit is a single patient, single use accessory (use only once).