This study was undertaken to inform clinicians as to the respective rescue medication drug delivery performance using the new portable spacer compared to the commonly recommended spacer as well as the MDI alone (assuming perfect coordination).
Performance of a Portable Spacer Compared to the Metered Dose Inhaler Alone and a Commonly Recommended Spacer
RATIONALE
Although Spacers (more correctly known as Valved Holding Chambers) have been shown to improve lung deposition, reduce side effects, and overcome challenges with inhaler coordination, they are often left at home due to their size and appearance. A new 2 in 1 spacer and protective case for the inhaler was developed in collaboration with patients to address some of the reasons for not using ‘On the Go’. This study was undertaken to inform clinicians as to the respective rescue medication drug delivery performance using the new portable spacer compared to the commonly recommended spacer as well as the MDI alone (assuming perfect coordination).
METHODS
Fine particle dose (FPD, µg<4.7µm) and fine particle fraction (%, %<4.7µm) were determined using an Andersen cascade impactor (CI) at 28.3 L/min in accordance with the pharmacopeial methodology. 5 actuations of Ventolin† or Atrovent† were delivered at 30s intervals to the device on test and the active pharmaceutical ingredient recovered from the CI was assayed by HPLC. Measurements were conducted on the pMDI alone, pMDI with AeroChamber Plus* Flow-Vu* spacer, and pMDI with a new portable spacer (AeroChamber2go*).
RESULTS
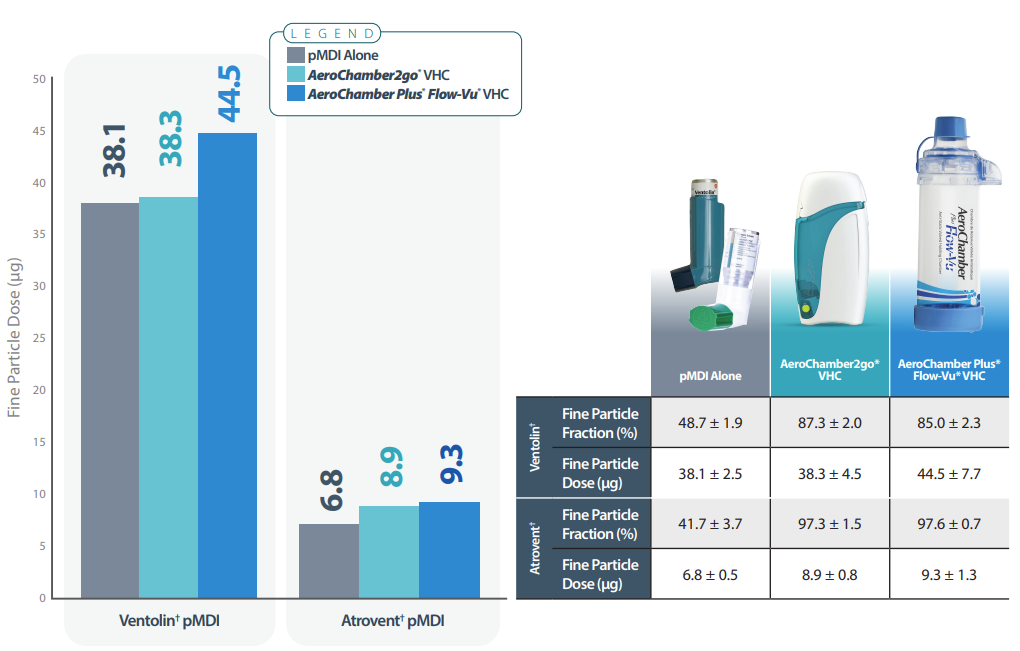
CONCLUSIONS
These in vitro data provide guidance to the clinician that the new AeroChamber2go* spacer provides only slightly lower fine particle dose (FPD) performance than the full-size standard spacer and similar FPD to that of the pMDI alone. Given the known real life coordination challenges using a pMDI alone, which may be even worse in a rescue situation, the portable spacer provides a more convenient alternative for those patients who currently do not use a spacer ‘On the Go'.
