INTRODUCTION
The delivery of inhaled medication to a young child from an MDI requires a valved holding chamber (VHC) with facemask. This system’s ability to deliver aerosol is dependent upon the interaction between facemask and face of the patient. The use of a model that includes soft face tissue simulation and an anatomically correct oropharyngeal airway is an effective means to evaluate MDI+VHC systems with a mask. We report a study in which several childmask VHCs were evaluated using a model of a 4-year-old child to deliver fluticasone.
METHODS
7 different VHC types were evaluated by breathing simulator (ASL 5000) programmed to simulate a coordination delay of 2 s before inhalation, followed by tidal breathing (tidal volume = 155- mL, I:E ratio = 1:2, rate = 25 cycles/min). Each VHC (n=3 devices/group) was used according to manufacturer’s instructions and applied to the anatomical model. The airway was coupled to the breathing simulator via a filter to capture drug particles that penetrated as far as the carina. 5-actuations of fluticasone (Flovent† 50 Evohaler) were delivered at 30-s intervals and recovered from specific locations in the aerosol pathway by HPLC. Comparisons were then made on drug delivery data looking at potential dose to the lungs for each pMDI/spacer.
RESULTS/DISCUSSION
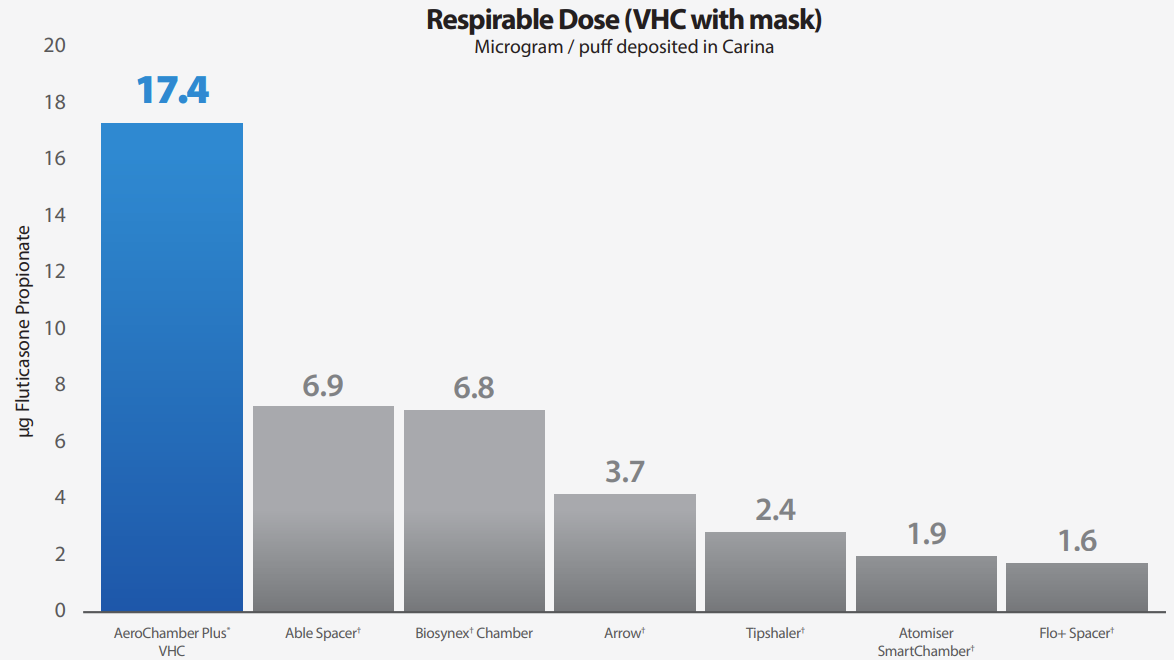
The mass of medication delivered to the ‘carina’ region of the model, that is the aerosol most likely to reach the patient’s lungs for the 7 devices ranged from 17.4±1.5µg for the AeroChamber Plus* to 1.6±0.6µg for the Flo+† Chambre d’inhalation. The differences in medication delivery can largely be explained by the mass of medication deposited within the VHC body, therefore, not available to the patient. The Tipshaler† Chambre d’inhalation retained 31.3±3.4µg compared to the AeroChamber Plus* with 9.6±1.0µg.
CONCLUSIONS
Significantly more fluticasone was delivered to filter/carina with the AeroChamber Plus* spacer. (un-paired t-test, p < 0.001). • Mask fit, spacer (shape, capacity, material) and valve design may account for the large differences in drug delivery. • Despite the spacers having similar application there are clear and likely to be clinically relevant differences in drug delivery performance between the devices. There should never be an assumption that spacers are all the same.
