INTRODUCTION
The optimal inhalation manoeuvre for passive DPIs is a short rapid intake of breath in order to provide sufficient energy to aerosolize and disperse the powder from the delivery source (blister package or reservoir) and, if the formulation is carrier based, to separate fine particles from the carrier particles so that they are capable of reaching the lungs.
In contrast, optimum delivery of inhaled medication delivered from a pMDI+VHC is achieved by a long, slow inhalation followed by a breath-hold.
METHODS
pMDI+VHC
The key instructions are to exhale then press the inhaler once at the beginning of a slow inhalation, then inhale slowly and deeply through the chamber until a full breath has been taken, followed by a breath-hold for 5 to 10 seconds before exhaling.
DPI
The key instructions are to exhale away from the inhaler; actuate it and immediately inhale once as deeply and strongly as you can (Turbuhaler† DPI) or long and deeply (Ellipta† DPI).
RESULTS
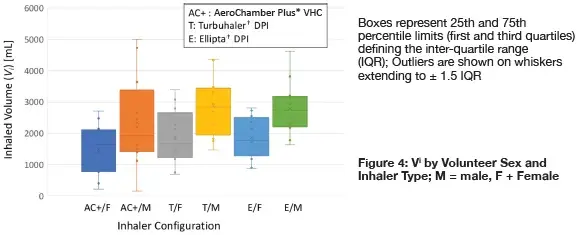
Individual measures of Vi segregated by volunteer sex and inhaler type:
Individual measures of PIFR segregated by volunteer sex and inhaler type:
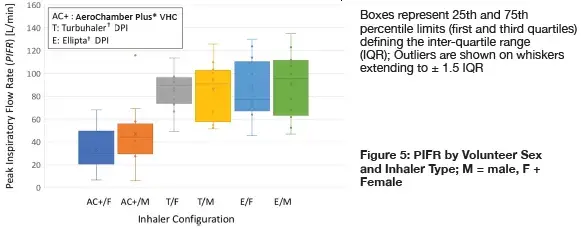
The large variability tended to mask any sex-based differences in either parameter. This much variability was expected even with healthy adults without lung disease, based on data from clinical studies with adult patients with asthma and chronic obstructive lung disease.2
Part of this variability can be accounted for in terms of how the user interacts with a particular inhaler (response to training).
Measures of PIFR were significantly greater for inhalation via either DPI compared with that via the VHC, (2-way t-test, p < 0.001) suggesting that volunteers were capturing the need to inhale more forcefully via the former devices, even though the instructions for use of the Turbuhaler† DPI (‘inhale as deeply and strongly as you can’) were different to those given for the Ellipta† DPI (‘take one long steady breath in’).
However mean values for males, especially for Vi, were greater than the corresponding data for the females as might be expected from sex-related anatomic considerations. Values of Vi for the males using the Turbuhaler† DPIs were statistically comparable to the corresponding measures from the females in the cohort (2-way t-test, p = 0.13). Corresponding values for the males using the Ellipta† DPI were significantly larger (p = 0.008). Values of PIFR for the same comparison between DPIs were similar (p =0.66).
Sex-based differences in either parameter for the VHC configuration were not statistically different (p = 0.083).
CONCLUSION
A limitation of this study is that all subjects were healthy, relatively young, educated, and without diagnosed lung disease, and therefore capable of understanding the patient leaflet and physically making the required manoeuvres. The extent of training given involved the volunteer reading and familiarizing him/herself with the patient instructions.
Further studies are planned involving the evaluation of medication delivery with inhalation profile variability. For instance, it would be interesting to see if those volunteers having extremely high values of PIFR would incur excessive oropharyngeal deposition because of the greater inertia of the particles leaving the DPI.
With the passing of the pandemic, additional work will look to address the impact of morbidities in particular asthma and chronic obstructive lung disease on medication delivery in association with inhalation capability.
1 Suggett JA, Nagel MW, Mitchell JP. Assessment of naïve inhaler user inhalation profiles and the impact on subsequent aerosol performance : Comparison of a dry powder inhaler (DPI) and a pressurized metered dose inhaler (pMDI) with valved holding chamber (VHC) using the same active pharmaceutical ingredients (APIs). (Abstract). Presented at: Drug Delivery to the Lungs-2019. The Aerosol Society, Edinburgh, UK. 2019: 30; pp. 97-100 2 Azouz W, Chetcuti P, Hosker HSR, Saralaya D, Stephenson D, Chrystyn H: The inhalation characteristics of patients when they use different dry powder inhalers. J Aerosol Med Pulmon Deliv 2015; 28(1): pp 35-42.). MD-230A-1122
