RATIONALE
The use of a model that includes soft face tissue simulation and an anatomically correct oropharyngeal airway is an effective means to evaluate MDI+valved holding chamber with a mask. We report a study in which several child-mask spacers (n=5/group) were evaluated using a model of a 4-year-old child.
METHODS
Each spacer with mask was attached to the anatomical model and the airway coupled to a breathing simulator (ASL5000) via a filter located at the exit to capture drug particles that penetrated as far as the carina. The breathing simulator was programmed to simulate a coordination delay of 2 s before inhalation, followed by tidal breathing (tidal volume = 155-mL, I:E ratio = 1:2, rate = 25 cycles/min). 5-actuations of Flovent† 50 were delivered at 30-s intervals and recovered from specific locations in the aerosol pathway and assayed by HPLC.
RESULTS
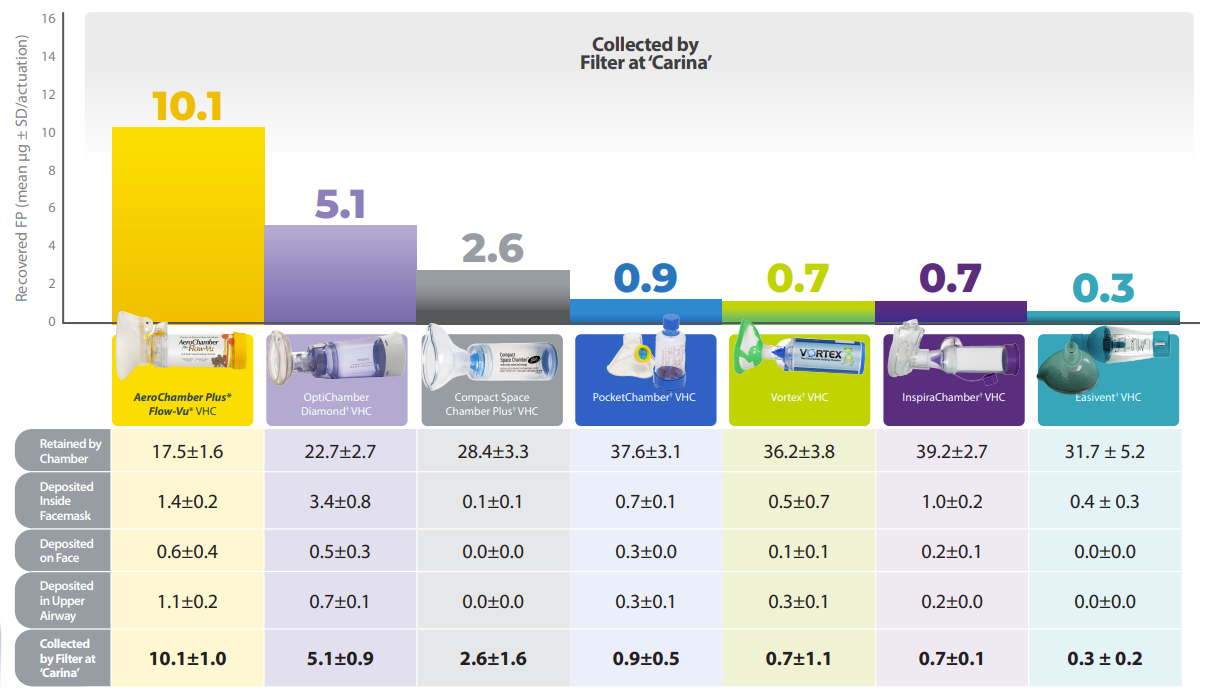
CONCLUSIONS
•Significantly more FP was delivered to the filter/carina with the AeroChamber Plus* Flow-Vu* spacer than with any of the other spacers (un-paired t-tests, p < 0.001).
•Mask fit, spacer (shape, capacity, material) and valve design may account for the large differences in drug delivery.
•Clinicians need to be aware that not all spacers are the same and will potentially deliver significantly different amounts of drug.
