Spacers Are Not Interchangeable - Aerosol Evaluation of Online Knock-Off Devices
Spacers Are Not Interchangeable - Aerosol Evaluation of Online Knock-Off Devices
RATIONALE
Online marketplaces offer convenience and a vast array of options making them an attractive choice for purchasing medical devices. However, spacers are an integral part of the pMDI delivery system, and this convenience comes with inherent dangers and risks that consumers must be aware of. The primary concern lies in the lack of regulation and oversight in online marketplaces, leading to the availability of counterfeit, substandard, and potentially dangerous products.
METHODS
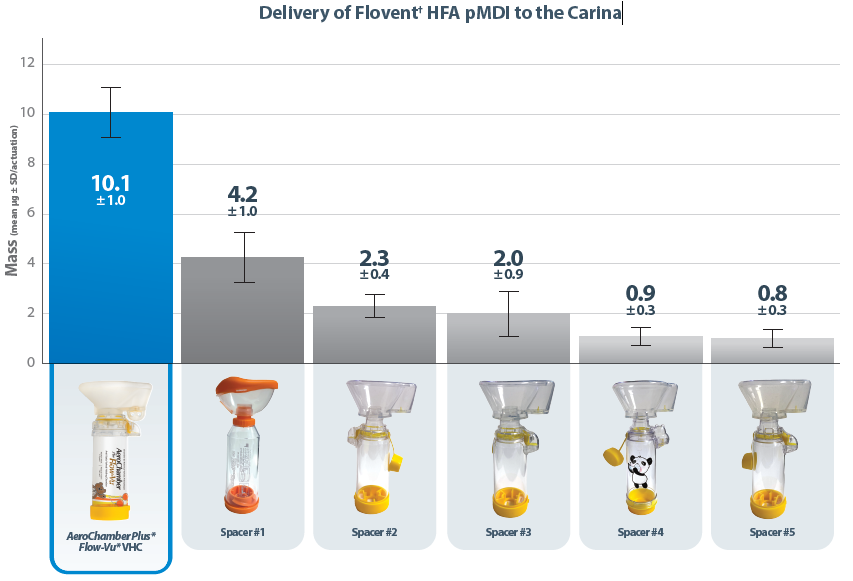
- Chambers were purchased from Amazon.ca and evaluated by breathing simulator mimicking a coordination delay of 2 seconds before inhalation, followed by tidal breathing (tidal volume = 155-mL, I:E ratio = 1:2, rate = 25 cycles/min).
- Each spacer (with mask) was attached to an anatomical model of a 4-year old child face and the airway coupled to the breathing simulator via a filter located at the exit to capture drug particles that penetrated as far as the carina.
- 5-actuations of fluticasone propionate (FP, Flovent† 50) were delivered at 30-s intervals and recovered from specific locations in the aerosol pathway by HPLC-UV spectrophotometry.
RESULTS
Delivery of Flovent† HFA pMDI to the Carina
 |
CONCLUSION
Developing a mobile health application using validated survey frameworks provides a systematic and user-centric approach that can help address the needs, desires, and expectations of patients. Developers can create an application that enhances disease management, improve patient outcomes, and promote self-care. In this case, the survey enabled the identification of a number of priority areas which were able to be followed up with patients and HCPs with the intent of making further improvements.
MD-326A-1023 ® trademarks and registered trademarks of Trudell Medical International (TMI). † trademarks of their respective companies. ©TMI 2023.
